Can mNGS Replace Culture?
In microbiology and infectious-disease work, culture has been the gold standard for over a century. But now, metagenomic next-generation sequencing (mNGS) is becoming more common. The question: can mNGS truly replace culture in diagnostics and research?
What are Culture and mNGS?
- Culture means growing microorganisms (bacteria, fungi, sometimes parasites) on media (plates, broth) under lab conditions. After growth, you identify the organism and often measure antibiotic sensitivity.

- mNGS (metagenomic next-generation sequencing) is a culture-independent method (NCBI). You extract all nucleic acids from a specimen, sequence them (DNA or RNA), then match the sequences to databases of known organisms. This allows you to detect bacteria, viruses, fungi, and parasites all in a single test.

Strengths of mNGS Over Culture
- Broad detection
Culture is limited: many pathogens cannot grow (or grow slowly) under standard lab conditions. mNGS can detect unculturable or difficult-to-grow organisms. NCBI - Faster turnaround (in some cases)
Culture often takes 24–72+ hours (or longer for slow growers). mNGS can sometimes give results within 24–48 hours, especially for simpler samples. - Resilience to prior antibiotic use
If a patient is already on antibiotics, culture may fail (because organisms are suppressed). mNGS may still pick up nucleic acids even if organisms are dead or nonviable. - Simultaneous detection
Culture is often targeted (you guess which organism and use selective media). mNGS is unbiased: you don’t need to decide ahead of time which pathogens to test. - Higher sensitivity in many studies
In several comparisons, mNGS had a higher positive detection rate than culture. For example, in neurosurgical CNS infections, culture detection was ~59.1%, but mNGS detection ~86.6%. In a respiratory / mixed infection study, the etiological diagnosis by mNGS was 78.9%, vs culture 20%. Discover - Better at rare, mixed, or fastidious pathogens
Some pathogens are rare or require special media (or are intracellular). mNGS can detect those where culture fails. - Potential value in antimicrobial stewardship
Faster, broader results could allow earlier targeted therapy and reduce misuse of broad antimicrobials.
Limitations & Challenges of mNGS
Despite the promise, mNGS is not a flawless replacement. It faces many practical and technical hurdles.
- Cost
mNGS is much more expensive per sample than culture in most settings. This limits its use in many labs or in routine diagnostics. - Turnaround time in practice
Although the sequencing itself can be fast, sample prep, bioinformatics, and interpretation add time and complexity. - Lower specificity / false positives
Because mNGS is sensitive, it may detect background contamination, commensal organisms, or DNA from dead organisms. Distinguishing clinical relevance is not trivial. - Lack of phenotypic antibiotic susceptibility data
Culture allows you to test which antibiotics kill or inhibit the organism (MIC, disk diffusion). mNGS gives genomic data, but inferring functional resistance often remains uncertain or incomplete. - Depth & detection limits
If pathogen load is very low, it may not reach sequencing detection threshold. Some samples have overwhelming host DNA, which drowns out pathogen reads. - Bioinformatics and database accuracy
The quality of reference databases, alignment algorithms, and filtering is crucial. Misannotation can lead to wrong identification. - Standardization, regulatory approval, validation
Many mNGS assays are still in academic or experimental stage. Standard operating procedures and regulatory frameworks are less mature than for culture. - Interpretation complexity
Physicians must integrate mNGS results with clinical data, imaging, lab tests, and patient history. Just because a sequence is found doesn’t always mean it’s the causative agent.
Comparing mNGS vs Culture in Real Studies
Here are some key findings from literature:
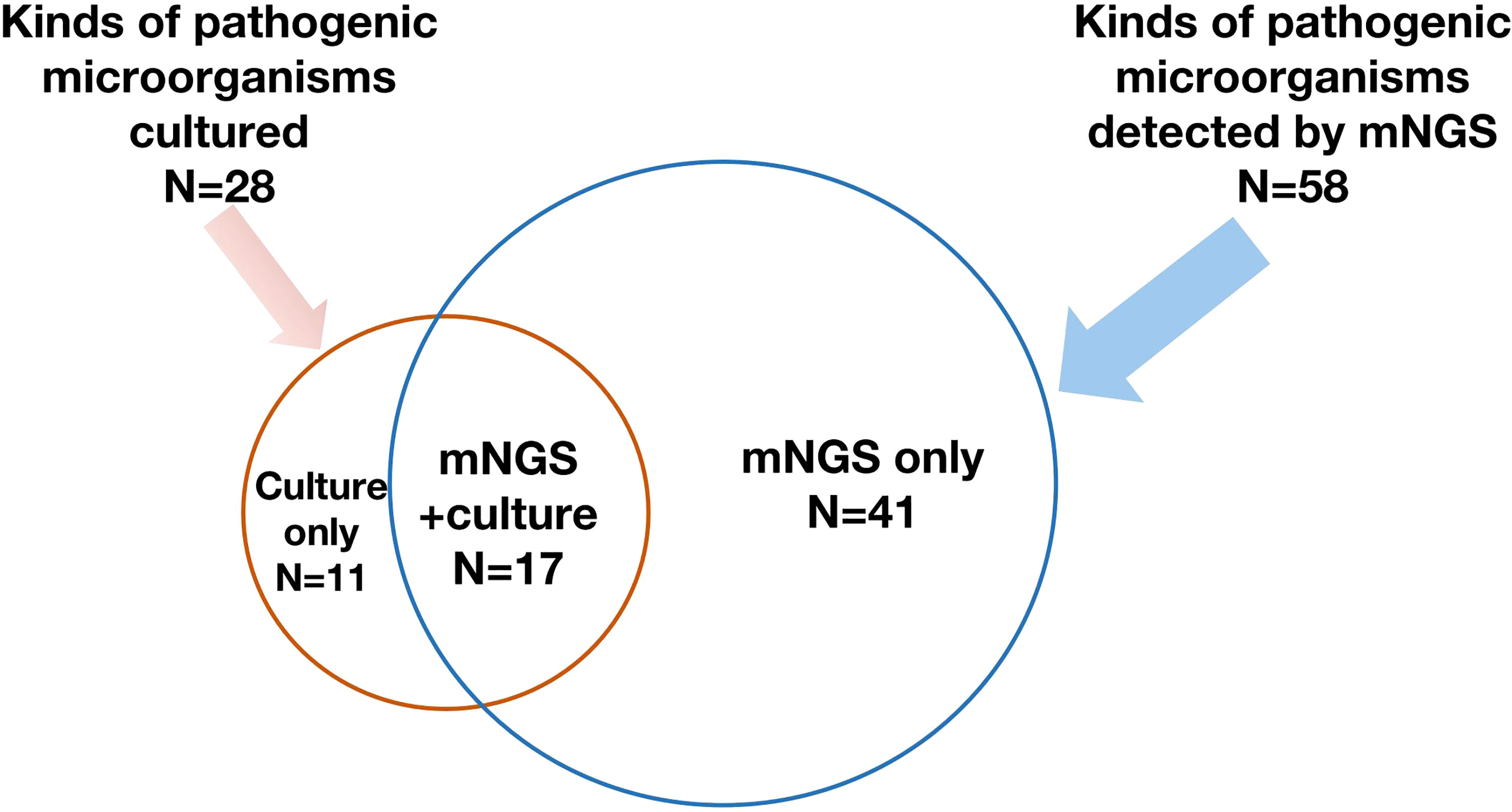
- In patients with suspected bloodstream infection, mNGS had a much higher detection rate (65.7%) than blood culture (13.1%) in one study. But overlap between the two was low (only ~12%) indicating both methods yield some unique findings. Discover more.
- In tissue (spinal) infections, a meta-analysis found pooled sensitivity of mNGS ~0.81, specificity ~0.75; for culture (tissue culture technique), sensitivity ~0.34, specificity ~0.93. That means culture is very specific but misses many pathogens, while mNGS catches more but may include more false findings. Learn more
- In critically ill patients with severe infections, one retrospective study found etiological diagnosis via mNGS 78.9% vs conventional microbiological testing 20%.
- Many studies also point out that combining mNGS with culture or conventional microbiological methods gives better overall diagnostic yield than either alone.
So, Can mNGS Replace Culture?
The short answer is: not completely, at least not yet. But in many use cases, it can greatly augment or partially replace culture.
Scenarios Where mNGS Could Be a Full or Major Replacement
- In cases where culture always fails (e.g. after antibiotics, fastidious organisms).
- For sterile-site infections where the background is low and the pathogen load is moderate.
- In research or diagnostic settings where cost is less limiting.
- In emerging pathogen detection, outbreak investigation, or when you suspect rare organisms.
Scenarios Where Culture Must Remain
- Whenever antibiotic susceptibility testing is critical.
- When you need live isolates (for further phenotypic study, vaccine, strain typing).
- In low-resource settings or routine labs where cost and infrastructure are constraints.
- Where regulatory or clinical workflows demand culture-based confirmation.
Hybrid / Integrated Model
The real future is hybrid workflows:
- Use mNGS early to cast a wide net and detect unexpected pathogens.
- Use culture for confirmatory, phenotypic, and susceptibility testing.
- Use mNGS results to guide culture media choices, enrich culture success.
- Use both to cross-validate and improve confidence.
Many studies argue that combining both methods yields the highest sensitivity, specificity, and clinical value.
Technical Considerations for Implementation
If a lab or diagnostic center wants to adopt or integrate mNGS, here are key technical points:
- Sample type matters : blood, CSF, tissue, BAL fluid, etc. Some are harder (high host DNA).
- Preprocessing & depletion : methods to reduce host DNA or enrich microbial DNA (e.g., host DNA depletion, differential lysis).
- Sequencing depth : more reads give better detection, but cost rises.
- Bioinformatics pipelines : filtering, alignment, taxonomy assignment, contamination control.
- Quality controls & negative controls : to guard against spurious detections.
- Reference databases : curated microbial genome collections (NCBI, RefSeq, pathogen libraries).
- Turnaround time optimization : pipeline automation, streamlined lab workflows.
- Validation & standards : cross-checking with known samples, proficiency testing.
- Clinician interface and reporting : clear reports, thresholds, actionable interpretation.
mNGS is transforming pathogen detection. It offers broad, fast, and sensitive detection capabilities that culture cannot match in many scenarios. But it still cannot fully replace culture in every case due to limitations in cost, phenotypic data, specificity, and infrastructure. The path forward is integration — use mNGS and culture together, complementing each other.
Over time, as sequencing costs drop, pipelines improve, and regulatory acceptance grows, mNGS may supplant culture in many areas. But for now, a hybrid or combined approach is the most robust strategy.
Recent Posts
-
Can mNGS Replace Culture?
In microbiology and infectious-disease work, culture has been the gold standard for over a century. …30th Sep 2025 -
Post-Translational Modifications: The Hidden Layers of Protein Regulation
Proteins are the workhorses of cellular biology, performing a wide array of functions that are essen …18th Oct 2024 -
Unveiling the Structure-Function Relationship in Proteins: Why Shape Matters
Proteins are fundamental biomolecules that drive virtually every biological process within an organi …18th Oct 2024